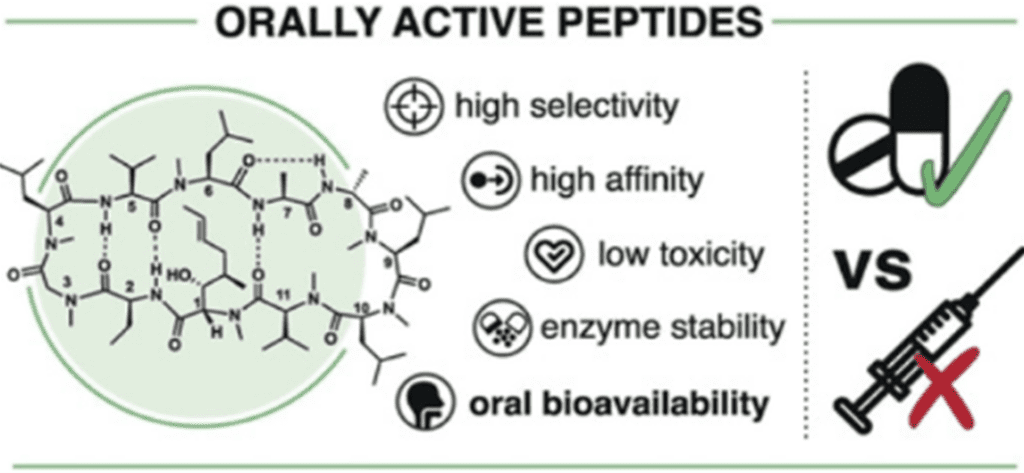
How to Take Peptides: What Are the Benefits of Oral Supplements?The Benefits of Oral Peptides Versus Injections
Peptides are smaller versions of proteins. Peptides are short strings of amino acids, typically comprising 2–50 amino acids. Amino acids are also the building blocks of proteins, but proteins contain more. Peptides may be easier for the body to absorb than proteins because they are smaller and more broken down than proteins. They can more easily penetrate the skin and intestines, which helps them to enter the bloodstream more quickly.
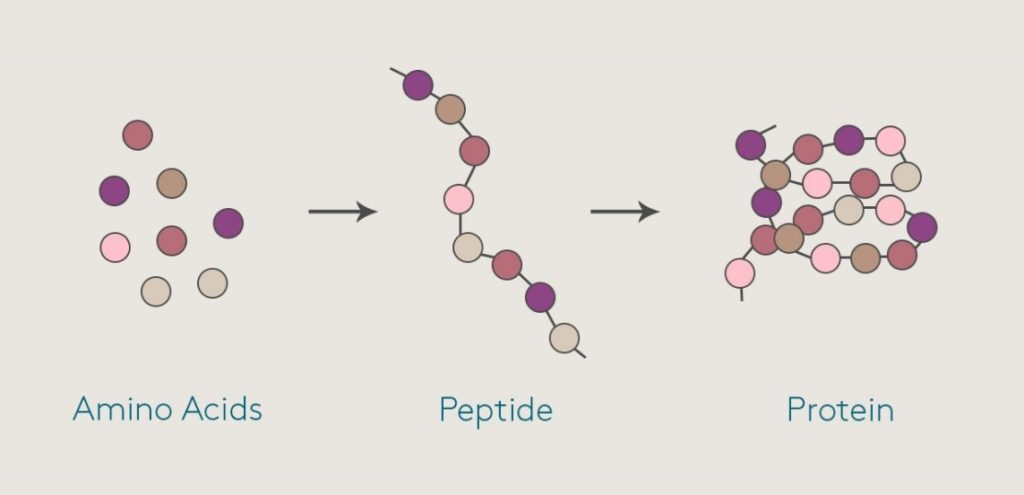
(Fig. 1. Different amino acid combine to make a peptide which leads to formation of a protein)
People may confuse peptides with proteins. Both proteins and peptides are made up of amino acids, but peptides contain far fewer amino acids than proteins. Like proteins, peptides are naturally present in foods. Due to the potential health benefits of peptides, many supplements are available that contain peptides that manufacturers have derived either from food or made synthetically.
Some of the most popular peptides include collagen peptides for anti-aging and skin health, and creatine peptide supplements for building muscle and enhancing athletic performance.
Research indicates that bioactive peptides may:
- Lower high blood pressure
- Kill microbes
- Reduce inflammation
- Prevent the formation of blood clots
- Improve immune function
- Act as antioxidants (1)
People often use peptides to try to achieve the following effects:
Slow down the aging process
Collagen is a protein in the skin, hair, and nails. Collagen peptides are broken down collagen proteins that the body can absorb more easily. Taking collagen peptides may improve skin health and slow the aging process. Some studies indicate that dietary food supplements that contain collagen peptides can treat skin wrinkles. Other research indicates that these supplements may also improve skin elasticity and hydration. (2)
Peptides may stimulate the production of melanin, a skin pigment, which may improve the skin’s protection against sun damage. Topical anti-aging cosmetics can also contain peptides, which manufacturers claim can reduce wrinkles, help skin firming, and increase blood flow.
Improve wound healing
As collagen is a vital component of healthy skin, collagen peptides may facilitate faster wound healing. Bioactive peptides can also reduce inflammation and act as antioxidants, which can improve the body’s ability to heal.
Research is currently ongoing into antimicrobial peptides, which may also improve wound healing. Having very high or very low levels of some antimicrobial peptides may contribute to skin disorders, such as psoriasis, rosacea, and eczema.
Prevent age-related bone loss
Animal research links a moderate intake of collagen peptides with an increase in bone mass in growing rats who also did running exercise. The study may point to collagen peptides being a useful way to counteract age-related bone loss. However, more research is necessary, especially on humans. (3)
Build strength and muscle mass
Some research on older adults indicates that collagen peptide supplements can increase muscle mass and strength. In the study, participants combined supplement use with resistance training. (4)
Creatine peptides may also improve strength and help to build muscle. While fitness enthusiasts have been using creatine protein powders for many years, creatine peptides are increasing in popularity. These particular peptides may be easier for the body to digest, which means they may cause fewer digestive problems than creatine proteins.
In this article, we discuss the potential benefits of oral peptide supplements.
Various routes of administration for peptide drugs
The various routes of administration for peptide drugs are:
- Parenteral delivery system
- Non-parenteral delivery system
- Oral route
- Nasal route
- Buccal route
- Ocular route
- Transdermal route
- Pulmonary route
- Rectal route
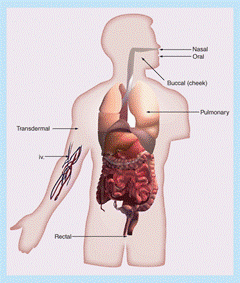
(Fig. 2. Different routes for administration of Peptides)
Parenteral delivery system
The parenteral delivery system includes intravenous, intramuscular, subcutaneous, intraperitoneal and intrathecal. It used to be the major route of choice for administering peptides, but due to high cost and pain during administration, new delivery systems came into existence for better patient compliance. (5) In the case of peptide delivery via injection (SC), interactions with the interstitial extracellular matrix (ECM) can impact systemic bioavailability of peptides (Figure 3).
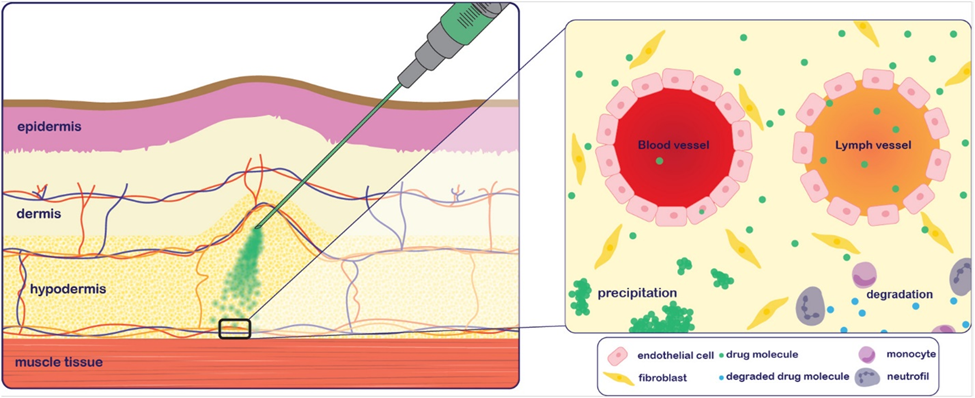
Fig. 3. Processes that may impact subcutaneous systemic bioavailability of a peptide after injection.
The interstitial space has a slightly negative charge due to the abundance of glycosaminoglycans such as
- Hyaluronan
- Chondroitin sulfate
- Heparin
Interactions with glycosaminoglycans and structural proteins (e.g., collagen and elastin) can hinder diffusion of the active compound, slowing absorption into systemic circulation. (6)
For example, insulin has been shown to bind to several ECM proteins such as type V collagen, vitronectin, and laminin. Like the mucosal membrane, factors such as size and lipophilicity will influence diffusion through the interstitial space.
Non parenteral delivery systems
Oral route:
The oral route is a better way to take peptides because of good patient compliance and acceptance Designing oral peptide and protein delivery systems has been a persistent challenge to pharmaceutical scientists because of their several unfavorable physicochemical properties including large molecular size, susceptibility to enzymatic degradation, short plasma half-life, ion permeability, immunogenicity, and the tendency to undergo aggregation, adsorption, and denaturation. Consequently, the absolute oral bioavailability levels of most peptides and proteins are less than 1%. The challenge here is to improve the oral bioavailability from less than 1% to at least 30-50%14. (12)
Designing and formulating a protein and peptide drug for delivery though GI tract requires a multitude of strategies such as:
Enteric coating
Enteric-coated capsules, tablets, or beads are used for a variety of oral formulations and often in combination with one or more additional oral strategies. In some cases, the enteric coating is used to protect the gastric mucosa from irritation caused by the therapeutic itself and in many cases to protect compounds where stability in the gastric environment is of concern. (8)
In the context of therapeutic peptides, enteric coatings protect against pH-induced degradation and enzymatic degradation by proteases found in the gastric milieu. Enteric coatings also provide the added advantage of delivering the therapeutic to the small or large intestine where higher concentration of the drug can be achieved due to lower intestinal fluid content, thus increasing flux and apparent permeability.
Enteric coatings comprise polymers that remain insoluble in the acidic pH of the stomach and become soluble when the pH increases in the intestines. For example, Eudragits are copolymers of methylmethacrylate and ethylacrylate with methacrylic acid that have carboxylic acid groups that ionize at the pH range of 5–7. (9)
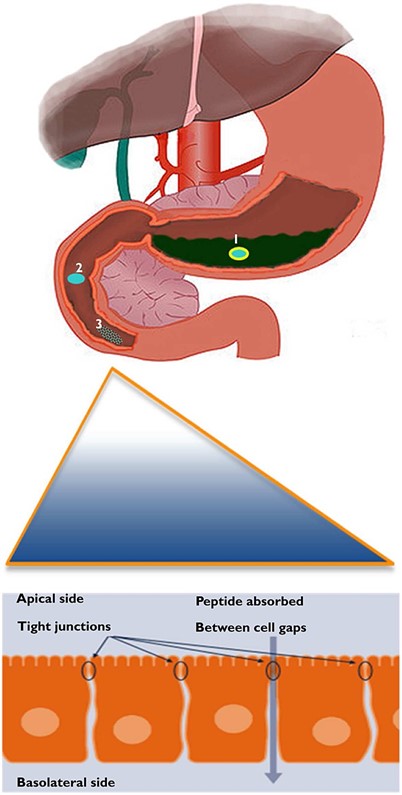
Fig. 4. Enteric coated peptide remains stable in the stomach.
Devices to Overcome Oral Delivery Barriers
In addition to formulation excipients, delivery devices can be used to overcome oral delivery barriers. Capsules designed appropriately can take advantage of the GI physiology to achieve the desired retention and transit times. RaniPill™ from Rani Therapeutics is one of the more advanced enteric-coated pill devices in clinical evaluation that injects directly into the wall of the jejunum. They demonstrated similar AUC for insulin delivered orally via the RaniPill™ compared to SC. Recently, the company announced positive phase I data for oral octreotide delivery achieving greater than 70% oral bioavailability.
Device design is of paramount importance as factors such as capsule size and ingestability must be in line with patient preferences. While many of these devices require the use of large capsules (28 × 11 mm), limitation of low-dose capacity is still a constraint; only 100 μg of octreotide was dosed in the recent clinical evaluation of RaniPill™. These design constraints can be challenging as they impose limitations on volume and concentration, which requires additional formulation development, and potentially limits patient acceptance for groups who have swallowing issues. (10)
Local delivery to the gastrointestinal tract
While there is much interest in the development of oral peptide formulations that achieve therapeutic systemic exposure, local delivery of peptides to the GIT has been successfully applied to inflammatory disease and bacterial infections. Here, the therapeutic peptide need not permeate across the intestinal membrane, but rather act on cells in the local microenvironment (i.e., resident immune cells or exogenous bacteria).
Protection of the peptide from degradation is the key factor in formulation development for these peptides. Some approved peptides for local GIT delivery include vancomycin, linaclotide, and plecanatide. Sublimity Therapeutics is currently clinically evaluating the SmPill® technology, which comprises cyclosporine encapsulated in enterically coated minispheres. The coated minisphere technology prevents drug release and absorption in the small intestines, allowing the therapeutic to be active locally in the colon. (11)
Nanoparticles
Nanoparticles have emerged as potential drug delivery systems and are used for targeting of therapeutic peptides to particular organs/tissues via the peroral route of administration. Significant work is being done to modify the nanoparticles for better understanding of the factors affecting muco-adhesion and their subsequent uptake by the GI tract. Florence and group have reported the role of various biodegradable polymers in increasing the bioavailability of nanoparticles.
In one of the studies performed by this group, approximately 10% of orally administered invasin-coated latex nanoparticles (500 nm in size) were found in the systemic circulation of rats. Their work suggested the existence of optimum colloidal size for efficient entrapment of nanoparticles within the mucous and for epithelial cells to grip the particulates to trigger endocytic events. (13)
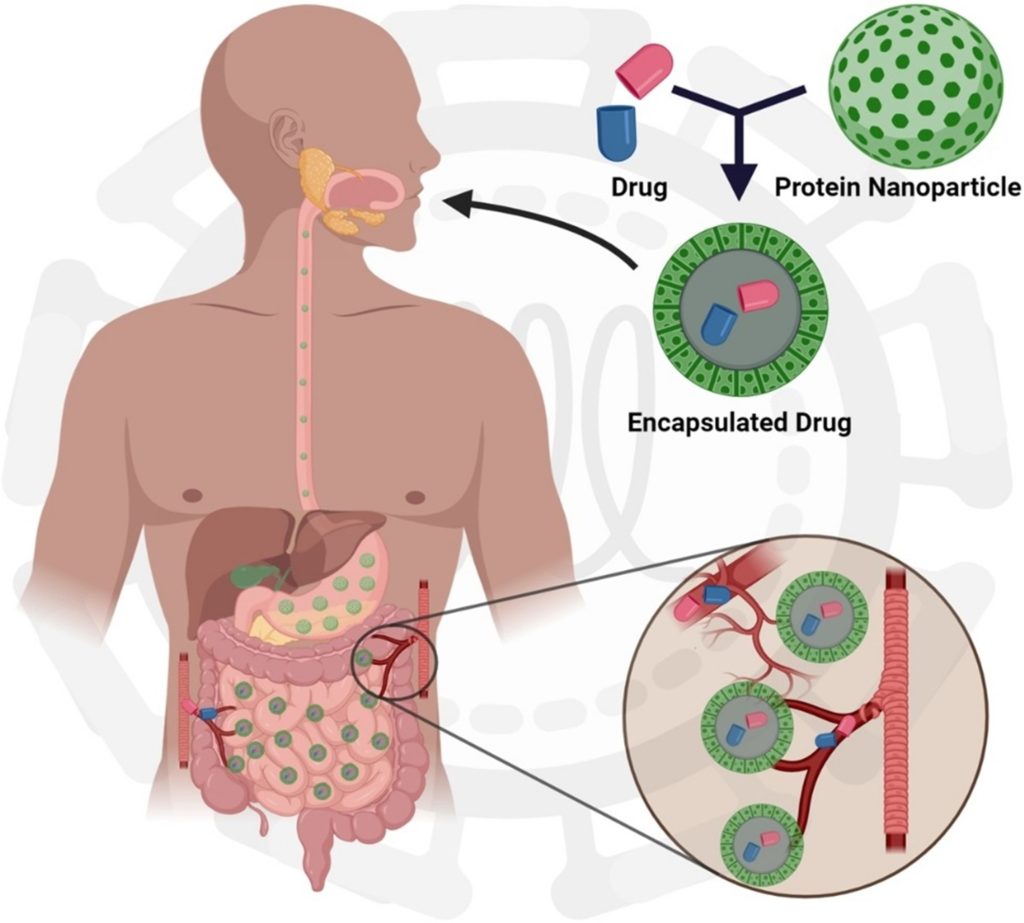
Fig. 5. Effective administration of peptide drugs via nanoparticles
Benefits of taking peptides via oral route:
Early in practice, it was considered useless to take oral proteins for anything besides basic nutritional support. The presumption was that they would be digested down to their component amino acids.
The finding that enzymes were orally absorbed was a major step forward. For example, it allowed the beginnings of a scientific basis for the work by Nicholas Gonzalez, MD, using pancreas for pancreatic cancer.
Recently, that work has been expanding to other oral polypeptides. For example, an area that has gained a lot of attention is use of oral collagen. Once again, the presumption was that taken orally, the collagen would go through complete digestion down to its basic amino acids. But animal studies have shown that this is not the case. (14)
This concept has now evolved considerably and has been a game-changer in chronic fatigue syndrome and fibromyalgia syndrome practice. In a study, an oral porcine serum polypeptide extract resulted in dramatic improvement in complex and disabling health cases. This led to do a small open study using the extract. In the study, 60% of people improved, and they showed an average 69% in both energy and overall wellbeing, along with an average 14% increase in total IgG and IgG 1-4 protective antibodies in those who had low total antibody levels pre-study. (15)
Fig. 6. Benefits of taking the peptides via Oral Route
Disadvantages of the Intravenous Route
- Possible anaphylaxis
- Risk of infection
- Inconvenient to the patient
- Painful
- Expensive compared to other routes
- Risk of phlebitis or extravasation
- Requires trained medical/nursing staff to administer
- Once injected, the drug cannot be recalled
- Labor intensive and time-consuming e.g. may require calculating the dose, looking up the diluents to be used, checking for IV drug compatibilities, preparation of IV drug and administering the injection.
Advantages of the Oral Route for administration of peptides are as follows:
Till recently, injections (i.e. intravenous, intramuscular or subcutaneous route) remain the most common means for administering these protein and peptide drugs. Patient compliance with drug administration regimens by any of these parenteral routes is generally poor and severely restricts the therapeutic value of the drug, particularly for disease such as diabetes1. Among the alternate routes that have been tried with varying degrees of success are the oral, buccal4, intranasal5, pulmonary, transdermal, ocular and rectal.
Among these, the oral route remains the most convenient way of delivering drugs. Some advantages are:
- For the treatment of pediatric patients, including the avoidance of pain and discomfort associated with injections
- Elimination of possible infections caused by inappropriate use or reuse of needles.
- Oral formulations are less expensive to produce, because they do not need to be manufactured under sterile conditions.
- A growing body of data suggests that for certain polypeptides such as insulin; the oral delivery route is more physiological (18)
Peptides and proteins have a high safety profile and cause minor side effects in comparison to conventional small molecules. Moreover, synthetic engineering and recombinant strategies have strongly advanced from those used to generate the first approved molecules such as insulin. However, therapeutic peptides and proteins have several significant limitations as therapeutics. Peptides and small proteins are rapidly cleared from systemic circulation by renal clearance, thus their efficacy is limited by a short circulating half-life. Furthermore, they are rapidly degraded by peptidases and poorly absorbed from mucosal membranes such as the small intestinal mucosa. (21)
Consequently, peptides and proteins are mostly administrated via the parenteral route, which is inconvenient and sometimes even painful and risky. Formulation scientists therefore favor the development of non-invasive delivery systems. Among the various non-invasive routes of administration, oral drug delivery is the by far preferred one owing to the ease of administration and high patient compliance. As a result of the harsh GI environment, however, orally administered peptide and protein drugs face numerous challenges such as inactivation by harsh pH values, enzymatic degradation by GI peptidases, thiol/disulfide exchange reactions with endogenous thiols and poor membrane permeability. (22)
Nonetheless, the number of oral peptide and protein formulations that are in clinical trials or already launched is continuously increasing. In Table 1 an overview about these oral peptide and protein delivery systems is provided. Among them, lipid-based nanocarriers are currently not the most favored approach. However, this situation will likely change in future as substantial progress was made on these formulations for oral peptide and protein delivery within recent years. Since, numerous surfactants and lipids that are listed in the pharmacopeia can be utilized for lipid-based nanocarriers and many of them can be found in the inactive ingredients list of approved oral drug products provided by the FDA (23), a great flexibility for their design is provided resulting in various types of formulations. In particular oil-in-water nano emulsions, self-emulsifying drug delivery systems (SEDDS), solid lipid nanoparticles (SLN), nanostructure lipid carriers (NLC), liposomes and micelles are of relevance for oral peptide and protein delivery.
Due to the formation of hydrophobic ion pairs (HIP) the lipophilic character of peptides and proteins can be tremendously raised. Consequently, they can be dissolved in the lipophilic phase of these delivery systems (24), where they are protected towards GI peptidases and thiol/disulfide exchange reactions with endogenous thiols. In particular muco-inert lipid-based nanocarriers permeate the mucus gel layer reaching the absorption membrane in high quantities where their payload can enter the systemic circulation. As lipid-based nanocarriers can interact with cells in numerous ways including endocytosis, transcytosis and even fusion with the cellular membrane, these delivery systems have the potential to overcome even the epithelial barrier for therapeutic peptides and proteins very efficiently.
Moreover, lipid-based formulations can be easily combined with permeation enhancers such as bile salts and fatty acids (25) which are currently the most favored approach. This review provides an overview about the different barriers for oral peptide and protein delivery and highlights the progress made on lipid-based nanocarriers as well as their key advances in order to overcome these barriers. Furthermore, we compare the strengths and weaknesses of lipid-based nanocarriers with other technologies for oral peptide and protein delivery.
Sustained Release of peptides is possible via oral route
Peptide/protein therapeutics have been significantly applied in the clinical treatment of various diseases such as cancer, diabetes, etc. owing to their high biocompatibility, specificity, and therapeutic efficacy. However, due to their immunogenicity, instability stemming from their complex tertiary and quaternary structure, vulnerability to enzyme degradation, and rapid renal clearance, the clinical application of protein/peptide therapeutics is significantly confined.
Though nanotechnology has been demonstrated to prevent enzyme degradation of the protein therapeutics and thus enhance the half-life, issues such as initial burst release and uncontrollable release kinetics are still unsolved. Moreover, the traditional administration method results in poor patient compliance, limiting the clinical application of protein/peptide therapeutics. Exploiting the sustained-release formulations for more controllable delivery of protein/peptide therapeutics to decrease the frequency of injection and enhance patient compliance is thus greatly meaningful. (16)
Cyclic peptides: More stable oral peptides for Crohn’s disease and Ulcerative Colitis:
Several peptides are used as drugs such as insulin, which controls the metabolism of sugar, and cyclosporine, which suppresses organ rejection after transplants. More than 40 peptides are already approved as drugs, generating revenues in the billions. There are several hundreds of peptide-based medications currently in clinical trials.
But almost none of these drug-peptides can be taken orally. Since peptides are an important part of food, the stomach and intestines harbor countless enzymes that can degrade them, meaning that most peptide-based medications do not survive the passage through the gastrointestinal tract.
Hope to generate more stable oral peptides came from “cyclic” peptides, whose ends are joined together by chemical bridges making them more stable than linear ones because their backbones are less flexible and thus harder to attack by enzymes. In 2018, a research group has developed a peptide format termed double-bridged peptides, where peptides are cyclized by two chemical bridges that provide even higher stability. Despite its success, most such peptides were not sufficiently stable to survive the enormous enzymatic pressure found in the gastrointestinal tract.
Now, this research group has developed a new method that identifies among billions of double-bridged peptides those that bind a disease target of interest and survive enzymes of the gastrointestinal tract. The method is published in Nature Biomedical Engineering, and involves three steps.
First, billions of genetically encoded random peptide sequences are cyclized by two chemical bridges that impose conformational constraints onto the peptides’ backbones so that they are more difficult to attack by enzymes. Second, this library of peptides is exposed to enzymes from cow intestine to eliminate all those peptides that are not stable. In the third and last step, the scientist dip target proteins into the pool of surviving peptides to fish out those that bind to the wanted disease target.
With this method, the researchers have succeeded for the first time in evolving target-specific peptides that can resist breakdown in the gastrointestinal tract. For example, they gave mice a lead peptide that inhibits thrombin — an important anti-thrombosis target — in the form of a pill. The peptide remained intact in the stomach and intestines, and even though it reached the blood stream in rather small quantities, most of it remained fully intact across the entire gastrointestinal tract. This is a key step towards engineering oral peptide drugs.
This research group is now applying the new method to develop oral peptides that act directly on gastrointestinal targets, meaning that they don’t need to travel into the blood stream. They are focusing on chronic inflammatory diseases of the gastrointestinal tract like Crohn’s disease and ulcerative colitis as well as bacterial infections. They have already succeeded in generating enzyme-resistant peptides against the interleukin-23 receptor, an important target of these diseases, which affect millions of patients worldwide without any oral drug available. (17)
Oral delivery: making peptides palatable
Oral delivery is a tantalizing double-edged sword for the biotech industry. On the one hand, it’s the most desirable route for getting drugs into the body, because it’s so convenient. Yet the oral route is also the most hazardous to the health of the peptides and proteins themselves, and probably will be the most difficult to achieve. In fact, a decade ago oral peptide delivery was considered fantasy.” And even today, though companies are plunging into the area, their early efforts have generated as much skepticism as excitement. The problem is that proteins can’t be taken in ordinary pills or capsules because their protective coatings break down in the stomach, exposing their contents to protein-splitting enzymes before they can be absorbed into the bloodstream.
The trick, then, is to find a way to camouflage peptides and proteins long enough to slip them past the digestive enzymes to the intestinal lining, where chances for absorption are greatest. Several companies think they can pull off that trick by protecting proteins from digestive enzymes by encapsulating them in microspheres-tiny particles, ranging in size from 50 nanometers up to 20 microns, made with any of a variety of inert materials, including synthetic and natural polymers. For example, InnoVet Inc., in West Palm Beach, Florida, working with Matrix Bioscience in Research Triangle Park, North Carolina, is experimenting with a natural fatty acid “biopolymer” to administer insulin orally; they say that they have had some success in rodents.
Alkermes Inc., in Cambridge, Massachusetts, is using the corn protein zein to deliver its peptides. And, as offbeat entries, there are the “proteinoids,” groups of unusual branched polymers, made entirely of amino acids. Emisphere Technologies, which holds the worldwide core patent on proteinoid technology, is experimenting with these unusual polymers as peptide protecting microspheres. Lots of people are skeptical about how well the microspheres will ever work. The mechanisms by which they might deliver peptides through the intestinal lining are not well understood, and so far, in animal studies, generally less than 10% and typically less 15 than 5% of the administered dose gets into blood. Nonetheless, some big players are hovering around the microsphere experiments, willing to risk significant R&D dollars on the outcome. (19)
Peptide sprays to stimulate weight loss
A quick spray on the tongue with an oral spray containing a compound that naturally occurs in the body could be all it takes to curb appetite and spark weight loss. And unlike oral weight-loss sprays currently on the market, this one holds the promise of being backed by medical science.
Results from a new University of Florida study show that an oral spray containing a peptide responsible for signaling fullness stymies obesity in animals and does not cause negative side effects as it does when the peptide is injected.
According to an associate professor of cellular and molecular therapy in the UF College of Medicine department of pediatrics, the chemical, called peptide YY, occurs naturally in the body and is released after eating.
When mice were treated with a solution of this peptide using a simple spray, with one puff they will consume less food and they will start losing weight. The implications were very simple: If you put peptide YY in a spray or gum and you take it half an hour before dinner, you will feel full faster and consume less food. It could be just a 5 or 10 percent difference, but it is enough to stimulate weight loss.
More than one-third of adults in the United States are obese, according to the Centers for Disease Control and Prevention. The results of the study, published in the Nov. 20 issue of The Journal of Neuroscience, could lead to an easy-to-use treatment that helps people eat less. (20)
Oral Peptide Products available on Kiya Longevity
BPC-57
BPC-157 is a peptide chain consisting of 15 amino acids. It is considered synthetic because this particular sequence does not exist in nature. It is derived from a protective protein found in the stomach.
Researchers have conducted numerous rodent studies on BPC-157 that show it has protective effects extending beyond the stomach and intestinal tract. BPC-157 has been shown to benefit ulcers in the stomach, intestinal damage such as fistulas and inflammatory disorders, bone and joint healing and growth rates, and organ damage. It also has some influences on the brain. Researchers have observed marked protective effects when BPC-157 is administered to rats alongside a research toxin or damaging surgical procedure.

More research is needed to clarify whether BPC-157 has multiple mechanisms of action, but current research suggests BPC-157 influences several muscle growth factors usually involved in angiogenesis (the production of blood vessels) and other factors involved in regeneration following damage. (26)
If you are taking BPC-157 orally, you don’t need to worry about precisely what volume to take. Just swallow one pill a day or whatever dosage as instructed on the box.
BPC 157 oral spray is another option. Oral administration has benefits for reducing brain inflammation, accelerating neuron repair, and fighting off allergy and mold toxicity symptoms. The effect is localized first and then systemic so although the absorption is much less, it’s quite effective for addressing the problem. Capsules are the least effective regarding absorbability, but if you are seeking to treat gut issues, the effect is seen to be effective. (26)

You can get the BPC-157 Body Protection Compound from Kiya Longevity. Our immediate-release capsules are 500 mcg each and are designed specifically for fast absorption. Whether you’re looking to boost your gut health or heal a specific injury, Kiya Longevity’s BPC-157 supplement allows you to get the full benefits of this amazing peptide.
Comparing the Bioavailability: Oral vs. Injectable BPC-157
The method of administration plays a crucial role in BPC-157’s bioavailability. Oral BPC-157 must pass through the digestive system, which can significantly reduce the amount that successfully enters the bloodstream. During digestion, some of the peptides may be broken down, which can impact its effectiveness.
In contrast, injectable BPC-157 is delivered directly into the bloodstream, bypassing the digestive tract. This often results in higher bioavailability, meaning more of the peptide remains intact and active, leading to potentially quicker results at lower doses.
To summarize, while oral BPC-157 can be effective, it requires larger doses and may take longer to exhibit noticeable effects than its injectable counterpart. This is due to the challenges faced in maintaining high bioavailability through the digestive process.
Differences Between Oral and Injectable Forms of BPC-157
Understanding the differences between oral and injectable forms of BPC-157 is crucial when deciding the best option for your needs.
Efficacy
Injectable BPC-157 generally proves more effective due to its direct absorption into the bloodstream. This method bypasses the digestive system, limiting the effectiveness of oral supplements. As a result, injections tend to deliver more consistent and reliable results.
Speed of Action
For those seeking immediate relief, injectable BPC-157 is typically a better choice as it works faster than its oral counterpart. Its immediate entry into the bloodstream allows for quicker results, whereas the oral form might take more time to manifest noticeable effects.
Duration of Effects
The effects of injectable BPC-157 tend to last longer. This is due to its enhanced bioavailability, meaning the body uses a greater proportion of the compound. This sustained action can be particularly beneficial for managing long-term conditions that require prolonged relief.
Convenience
When it comes to ease of use, oral BPC-157 is more convenient. It can be taken without the need for injection equipment or medical assistance, making it a more straightforward option for many. However, those willing to undertake the additional effort of injections may benefit from the faster action and prolonged effects.
In summary, the choice between oral and injectable forms of BPC-157 hinges on personal preference and specific treatment goals. If the priority is effectiveness and quick results, injections are likely the better choice, whereas oral administration offers convenience and ease.
When considering BPC-157 injections, it’s essential to weigh both the advantages and disadvantages to make an informed decision.
Pros of BPC-157 Injections
- Enhanced Absorption
Injections allow BPC-157 to enter the bloodstream directly, greatly increasing its absorption and efficacy compared to oral supplements. - Rapid Onset
The effects of the injection can be felt relatively quickly. This is particularly beneficial for addressing acute conditions that require prompt intervention. - Accurate Dosage
With injections, you can precisely control the dosage. This precision minimizes the potential for side effects and enhances therapeutic outcomes.
Cons of BPC-157 Injections
- Training Requirement
Administering injections requires some level of training. Learning the proper techniques is crucial to ensuring safety and efficacy. - Potential Discomfort
For some individuals, needle-based delivery can be uncomfortable. This might be a deterrent for those who are needle-averse. - Infection Risk
If the injection site isn’t adequately sterilized, there is a risk of infection. Proper hygiene practices are essential to minimize this risk.
Overall, while BPC-157 injections offer several significant benefits, they also come with challenges that should be considered. Whether or not this method is right for you depends on your comfort level with injections and your specific health needs.
Understanding the Onset of Action: Oral vs. Injectable BPC-157
When considering how quickly BPC-157 takes effect, it’s essential to highlight the differences between oral and injectable forms.
Injectable BPC-157:
- Rapid Onset: Injections deliver BPC-157 directly into the bloodstream, which allows the body to experience faster effects. This is especially beneficial when immediate relief from symptoms or acute conditions is needed.
- Targeted Delivery: Because injections bypass the digestive system, the peptide can act more directly and efficiently.
Oral BPC-157:
- Gradual Effect: Oral forms may require more time before effects are noticeable. This delay is caused by the need for digestion and absorption through the gastrointestinal tract.
- Convenience Factor: Despite the slower onset, oral administration provides a more convenient and non-invasive option for regular use.
Choosing between oral and injectable BPC-157 will ultimately depend on individual needs, aimed results, and other personal preferences or medical recommendations.
Understanding the Differences Between BPC-157 and PDA
When exploring healing supplements, two options often discussed are BPC-157 and PDA. While both are known for their healing properties, their distinct differences make them unique.
BPC-157: The Research Star
- Origin and Use: BPC-157 is a synthetic peptide derived from a protein found in the stomach. Primarily, it’s used in research settings rather than as a standard treatment for injuries or ailments.
- Regulatory Status: Currently, BPC-157 is not approved by major medical authorities for medical use, which means it’s typically unavailable through prescriptions.
PDA: A Prescription-Ready Option
- Legal Status and Prescriptions: Unlike BPC-157, PDA is legal for medical use and can be prescribed by healthcare professionals. This provides a level of safety and oversight that benefits patient care.
- Enhanced Longevity: One of the key features of PDA is its inclusion of arginate. This component extends the duration it remains active in the body, potentially increasing its healing effectiveness.
Treating Conditions with BPC-157 Injections
BPC-157 injections are particularly beneficial for addressing specific medical conditions due to their potent healing properties. Here’s a closer look at where these injections excel:
- Acute Injuries: When you sustain a sudden or severe injury, quick recovery is crucial. BPC-157 effectively speeds up the body’s natural healing processes.
- Post-Surgery Recovery: After surgery, the body must heal efficiently to regain full function. These injections help reduce recovery time and improve healing outcomes by promoting tissue regeneration.
- Severe Inflammation: BPC-157 is known for its anti-inflammatory properties, making it highly effective in reducing inflammation that causes discomfort and slows healing.
- Muscle and Tendon Repair: Athletes and active individuals often face muscle and tendon injuries. BPC-157 supports the rapid repair and strengthening of these tissues, helping you get back to your routine faster.
Whether you’re dealing with a fresh injury or recovering from surgery, BPC-157 injections provide a robust solution to enhance your recovery process.
Why Consult a Healthcare Provider Before Using BPC-157?
Deciding to use BPC-157 involves more than just reading about its potential benefits. Here’s why consulting a healthcare provider is crucial:
- Personalized Advice
Everyone’s health needs are unique. A healthcare provider offers tailored guidance, ensuring that BPC-157 aligns with your specific health requirements and goals. - Safety First
BPC-157, like any peptide or supplement, can have varied effects. Consulting with a medical professional helps identify possible interactions with your current medications or health conditions, making your usage safer. - Balancing Pros and Cons
A healthcare expert can provide a balanced view, helping you weigh the potential benefits against the risks based on your health profile. This informed approach empowers you to make educated decisions. - Regulated Usage
Dosage and duration of use should be carefully managed. A healthcare provider can help establish an effective and safe protocol, minimizing potential side effects.
Consultation with healthcare professionals is a comprehensive step that ensures your journey with BPC-157 enhances your health in the safest possible manner.
Can You Obtain a Medical Prescription for BPC-157?
In the United States, BPC-157 is not approved by the Food and Drug Administration (FDA), meaning healthcare professionals cannot prescribe it. While this peptide has garnered interest for its potential therapeutic uses, the lack of FDA approval limits its availability through traditional prescription channels.
Legal Alternatives:
Despite the restrictions on BPC-157, there are FDA-approved alternatives that some doctors might prescribe, which offer similar benefits. These alternatives, while not identical, may provide options for people seeking comparable therapeutic effects.
For those considering BPC-157, it’s important to consult healthcare providers on legal options that suit their medical needs. When exploring treatment possibilities, always prioritize safety and compliance with legal regulations.
Understanding Pentadecapeptide Arginate (PDA) and BPC-157
Pentadecapeptide Arginate, or PDA, is a specialized peptide designed to promote healing and recovery. It draws inspiration from BPC-157, a well-known peptide, but incorporates specific alterations to align with the rigorous safety and effectiveness requirements established by the FDA.
Key Features of PDA
- Legal and Prescribable: One of the standout benefits of PDA is its legal status. Unlike BPC-157, which remains in a legal gray area due to its classification as an unapproved drug, PDA can be legally prescribed by licensed healthcare providers. This means patients can access professional guidance when using PDA for therapeutic purposes.
- Enhanced Effectiveness: PDA is formulated with arginate, significantly extending its body presence. This prolongation enhances its therapeutic effectiveness, optimizing recovery outcomes.
Comparing to BPC-157
- Safety and Regulation: While both peptides are utilized for their healing properties, PDA meets strict FDA standards, whereas BPC-157 lacks formal approval. This distinction makes PDA a safer choice under medical supervision.
- Usage: Both are intended for similar applications; however, PDA’s legal status allows it to integrate seamlessly into treatment plans under medical guidance.
In summary, Pentadecapeptide Arginate emerges as a safer, more regulated alternative to BPC-157, providing promising healing benefits under the umbrella of legal medical practice.
How to Properly Store BPC-157 in Both Oral and Injectable Forms
Oral BPC-157:
- Store in a cool and dry environment.
- Please keep it away from direct sunlight.
- Ensure the storage area has consistent temperature levels to maintain potency.
Injectable BPC-157:
- Refrigerate to preserve its effectiveness.
- Ensure it is stored in a designated area within the fridge to prevent contamination.
- Avoid freezing, as this can compromise the solution.
General Tips:
- Always adhere to any storage guidelines provided by healthcare professionals.
- Check the product’s packaging for additional instructions or storage requirements.
- Taking these steps will help ensure the medication remains effective until it’s used.
KPV (Lysine, Proline, Valine)
KPV is a peptide that is naturally produced in the body. It is found in the hormone alpha-MSH, a relatively newer peptide that is an alpha-melanocyte-stimulating hormone. The latest research on hormones in this class has shown immune-modulating and anti-inflammatory effects. KPV has many uses in both auto-immune and inflammatory conditions.
This peptide comes as a cream, injectable, and oral capsule. The route of administration depends on the area to be targeted. KPV works very well as a topical cream for acne, eczema, and psoriasis. In an oral form, it can help with ulcerative colitis, irritable bowel syndrome, and Crohn’s disease. The injectable is used for an overall systemic anti-inflammatory effect.
Anti-Inflammatory
An overwhelming body of clinical evidence suggests that KPV exerts its anti-inflammatory solid properties through various important mechanisms. KPV exerts its anti-inflammatory function inside cells, where it inactivates inflammatory pathways. KPV can enter the cell and interact directly with inflammatory signaling molecules. It enters the cell’s nucleus, and once it’s there, it can inhibit the inflammatory substances and molecules. This leads us to the many benefits KPV has on the gut.
KPV can stop the proinflammatory mechanisms in intestinal epithelial and immune cells. It can interact directly with immune cells, which can reduce inflammation. KPV significantly decreased inflammation in colitis. It decreases the inflammatory response by inhibiting proinflammatory cytokine (molecule) synthesis and secretion. KPV may help in the case of IBDs through inhibited immune responses. (27)
Taken orally, KPV decreases pro-inflammatory cytokines, which can reduce the incidence of colitis.
Anti-Microbial
KPV also has antimicrobial effects against pathogens. Its antimicrobial effects were demonstrated on two major pathogens called S. aureus and C. albicans. In one study, KPV significantly inhibited S. aureus from forming colonies. KPV, with its anti-inflammatory and anti-microbial properties, can be very helpful for combatting these microbes and healing wounds. (30)

Wound Healing/Skin
Research in wound healing shows KPV can speed wound healing, reduce infection, fight inflammation, and lead to better cosmetic results. These benefits occur at physiologic concentrations which means KPV could help prevent infection in the setting of serious wounds like burns.
KPV is able to reduce the kind of inflammation that leads to a hypertrophic scar (keloid) formation. Administering KPV (a-MSH) in this setting leads to smaller scars and less drastic inflammatory response. Part of the benefit in reducing scar prominence appears to come from its ability to modulate collagen metabolism.
KPV has significant antimicrobial and anti-inflammatory properties especially in those with psoriasis. Psoriasis is a chronic autoimmune condition that causes the rapid build-up of skin cells and is usually treated using hydrocortisone. In people with psoriasis, KPV has been shown to limit symptoms of the condition, including itchiness, dryness, redness, peeling, and more. Therefore, KPV could be used for an extended period of time without risking the unwanted complications of long-term steroid therapy. (28)(29)
Can’t weight
Can’t Weight is a protein hydrolysate that acts on modulators of short-term food intake at the hypothalamic and digestive levels, as well as provides a reduction in the hunger hormone ghrelin. It has been shown to help lower calorie intake and reduce body fat mass.

Complex pathways modulate energy balance, involving appetite centers in the hypothalamus and hormonal signals of energy status released by the gut and the periphery. As glycemia levels lower we begin to feel hungry, and when the stomach empties it secretes ghrelin, the hunger hormone, which functions as a neuropeptide in the central nervous system. All these signals will activate the release of neuropeptide Y (NPY) in the hypothalamus and initiate the next food intake. Satiety and appetite are triggered by modulation of our levels of ghrelin. Can’t Weight acts as a satiety enhancer by its action of reducing ghrelin production and by a long-term regulation of optimal leptin levels. (31)
CerebroPep
A special formulation of porcine derived peptide blend brings the highest standard in quality and safety to support cognitive health and well-being. Similar to other porcine neuro peptides such a Cerebrolysin, which is administered through injection, CerebroPep marks the entrance of Integrative Peptides into the neuro and cognitive space without a needle.

This can aid in the repair and recovery of nerve cells within the brain, neuroprotective, improves cognition, memory, learning, creativity, and motivation. Used for treating concussions, Traumatic Brain Injury, Alzheimer’s, dementia, strokes, TIA, Parkinson’s, and Multiple Sclerosis.
It can be used for the following benefits:
- Improvements in brain function
- Improvements in cognitive focus and learning
- Improvements in memory and recall
- Improvements in synaptic connections (32)
Thymogen Alpha-1
Thymogen Alpha-1 contains a synergistic combination of immune-modulating, bioregulator dipeptides Thymogen and immune A2 (Lys-Glu).

Based on clinical trials, published literature, and through use of a new technology called metabolomics (measuring the metabolites of large numbers of chemical reactions), this combination is shown to replace, even outperform, the peptide thymosin alpha-1. (31)
TB4-FRAG-MAX
TB4-Frag Max is made with a specialized form of Thymosin Beta-4 Active Frag, shown through extensive research to have a high affinity binding to the cellular receptor resulting in much higher potency.
To further increase potency, TB4-Frag Max contains twice the amount of Active Frag per capsule, combined with a synergistic blend of absorbable bioactive thymic peptides, extracted naturally from bovine (sheep).

TB4-Frag Max contains the active fragment of the larger peptide Thymosin Beta. Thymosin Beta 4 (Tβ4) is a naturally occurring peptide.
Thymosin Beta is found in high concentrations in blood platelets, wound fluid and other tissues in the body. Tβ4 has been found to play an important role in protection, regeneration, and remodeling of injured or damaged tissues.
Thymosin Beta 4 is usually used for acute injury, surgical repair, and patients who were once athletes that accumulated injury over their lifetime. It is also used as a way to improve inflammation. (3)
Table 1. Oral peptide and protein delivery systems that are either in clinical trials or commercialized.
| Approach | Peptide/Protein | Dosage form | Comments | Company | Stage | Ref |
| Permeation enhancer | Insulin | Enteric-coated capsule | Formulation contains a mixture of solubilizer and permeation enhancer (aromatic alcohols) | Diabetology | Phase II | (34) |
| Insulin | Capsule | Bile salts were used as permeation enhancer and aprotinin as protease inhibitor | Oramed Pharmaceutics | Phase II | (35) | |
| Leuprolide | Tablet | Lyophilized leuprolide with maltodextrin, citric acid and acyl carnitine | Enteris BioPharma | Phase II | (36) | |
| Parathyroid hormone (PTH) | Tablet | 5-CNAC (8-(N-2-hydroxy-5-chloro-benzoyl)-amino-caprylic acid is used as permeation enhancer | Novartis | Phase II | (37) | |
| Salmon calcitonin | Tablet | 5-CNAC is used as permeation enhancer. The combination of 5-CNAC forms with salmon calcitonin insoluble carriers at low pH, avoiding enzymatic degradation in the GI-tract. | Nordic Biosciences | Phase III | (38) | |
| Salmon calcitonin | Enteric-coated capsule | * | Proxima Concepts Ltd | Phase III | (39) | |
| Salmon calcitonin | Tablet | * | Tarsa Therapeutics | Phase III | (40) | |
| Semaglutide | Tablet | SNAC (sodium N- [8-(2-hydroxybenzoyl) amino caprylate) is used as permeation enhancer) | Novo Nordisk | approved | (41) | |
| Lipid-based formulation | Cyclosporine | Capsule Solution | Self-emulsifying drug delivery system forming in contact with GI fluid a homogeneous microemulsion | Novartis | approved | (42) |
| Exetanide | Capsule | Formulation consists of omega-3 fatty acid as a carrier and soy bean trypsin inhibitor as a protease inhibitor and sodium EDTA as a permeation enhancer | Oramed Pharmaceutics | Phase I | (43) | |
| Insulin | Gel capsules | Liposomes with a size < 150 nm diameter loaded with insulin | Diasome | Phase II | (44) | |
| Insulin | Enteric-coated tablet | Micelles based on medium-chain fatty acid glycerides | Novo Nordisk | Phase II | (45) | |
| Insulin | Solution (Buccal spray) | Micelles > 7 nm in size delivering insulin to the oral mucosa. | Generex | Phase III | (46) | |
| Octreotide | Capsule | Oily suspension based on transient permeation enhancer | Chiasma | approved | (47) | |
| Chemical modification | Dolcanatide | Tablet | * | Synergy Pharmaceuticals | Phase II | (48) |
| Desmopressin | Tablet | Certain L-amino acids are substituted by D-amino acids | Ferring Pharmaceuticals | approved | (49) | |
| Linaclotide | Capsule | Inhibitor cystine-knots (ICKs), also called knottins containing macrocycles formed by disulfide bonds between cysteines I and IV and II and V | Ironwood Pharmaceuticals | approved | (50) | |
| Nanoparticles with hydrophobic surface | Insulin | Capsule | Silica nanoparticles with hydrophobic surface and branched polysaccharide | Oshadi Drug Administration | Phase II | (51) |
| pH sensitive formulation | Plecanatide | Tablet | pH-sensitive formulation | Synergy Pharmaceuticals | approved | (52) |
Key Differences Between BPC-157 Oral Administration and Injections
Effectiveness
When considering efficacy, injectable BPC-157 generally takes the lead. Injections deliver the substance straight into the bloodstream, maximizing absorption and potency. In contrast, oral forms might be less effective as they pass through the digestive system, potentially reducing their impact.
Speed of Action
For those needing rapid relief, injectable BPC-157 often works more quickly. The direct introduction into the bloodstream allows for a faster onset of effects. Oral alternatives, however, may require more time to produce noticeable results, as the body must first process them.
Duration of Relief
Regarding how long the effects last, injections typically provide a more sustained impact. This is due to the higher bioavailability when injected, which can offer consistent relief over time. Oral forms may need more frequent dosing to achieve similar outcomes, as the effects can wear off sooner.
Convenience and Ease
From a convenience standpoint, oral administration is undeniably more straightforward, requiring no special skills or equipment. However, albeit more involved, injections are favored by those who prioritize faster results.
Personal Preference and Needs
Choosing between oral and injectable BPC-157 should align with personal circumstances and therapeutic goals. While injections offer speed and longer-lasting effects, oral forms may be preferred for their simplicity and ease of use.
FAQs: Oral Peptides vs. Injections – What’s Right for You?
What’s the main difference between oral peptides and peptide injections?
It’s the delivery method! Oral peptides come in capsules or liquids, while peptide injections require direct delivery into the body. This affects how quickly they work and their stability.
Are oral peptides as effective as peptide injections?
This is an active area of research. Traditionally, peptide injection therapy was considered more reliable due to concerns about oral peptides breaking down in the gut. However, new technologies using scientifically formulated peptides with protective coatings or additions like medium-chain fatty acids might improve oral absorption.
What are the advantages of oral peptide therapy peptides?
- Convenience: Easier to self-administer, no needles required.
- Less Invasive: A good option for people who are needle-averse.
- Potential Cost-Effectiveness: This may be more affordable in the long term in some cases.
When are peptide injections still the preferred method?
- Precision: Injections deliver a more controlled dose directly into circulation.
- Peptide Stability: Some peptides are not viable when taken orally due to their structure.
- Maximum Potency: For specific therapeutic goals and peptides, injections might offer the best chance of maximum effect.
Do oral peptides have any specific use cases?
Emerging research is exploring their potential for areas like:
- Supporting Gut Health: Certain peptides might be beneficial for gut lining repair
- Boosting Growth Hormone: Promoting natural growth hormone release with human growth hormone-releasing peptides
- Skin Health: Stimulating collagen and elastin production
How do I decide which peptide therapy option is right for me?
Always consult with a qualified healthcare provider. They’ll assess your specific needs, the type of peptide therapy, and help you choose the most appropriate delivery method based on your health goals and individual circumstances.
References
- Chakrabarti, S., Guha, S., & Majumder, K. (2018). Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients, 10(11), 1738.
- Proksch, E., Schunck, M., Zague, V., Segger, D., Degwert, J., & Oesser, S. (2014). Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin pharmacology and physiology, 27(3), 113-119.
- Takeda, S., Park, J. H., Kawashima, E., Ezawa, I., & Omi, N. (2013). Hydrolyzed collagen intake increases bone mass of growing rats trained with running exercise. Journal of the International Society of Sports Nutrition, 10(1), 1-9.
- Zdzieblik, D., Oesser, S., Baumstark, M. W., Gollhofer, A., & König, D. (2015). Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. British Journal of Nutrition, 114(8), 1237-1245.
- Jain A, Gulbake A, Peptide and protein delivery using new drug delivery systems, Critical Reviews in Therapeutic Drug Carrier Systems, PubMed 2013, 30, 293-329.
- Improving the outcomes of biopharmaceutical delivery via the subcutaneous route by understanding the chemical, physical and physiological properties of the subcutaneous injection site. J Control Release, 182 (2014), pp. 22-32
- C. Maderuelo, J.M. Lanao, A. Zarzuelo
- Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur J Pharm Sci, 138 (2019)
- S. Thakral, N.K. Thakral, D.K. Majumdar, Eudragit (R): a technology evaluation. Expert Opin Drug Deliv, 10 (2013), pp. 131-149
- Ganesh, A. N., Heusser, C., Garad, S., & Sánchez-Félix, M. V. (2021). Patient-centric design for peptide delivery: Trends in routes of administration and advancement in drug delivery technologies. Medicine in Drug Discovery, 9, 100079.
- A study evaluating the efficacy and safety of ST-0529 in subjects with moderately to severely active ulcerative colitis, (2020)
- 14. Vincent HL Lee, Satish DK, George MG, Werner R. Oral route of protein and peptide drug delivery. In: Lee VH, editor. Peptide and protein drug delivery. New York: Marcel Dekker; 1991. pp. 691–738.
- Florence AT, Hussain N. (2001). Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev 50:69–89
- Watanabe-Kamiyama, M., Shimizu, M., Kamiyama, S., Taguchi, Y., Sone, H., Morimatsu, F., … & Komai, M. (2010). Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. Journal of agricultural and food chemistry, 58(2), 835-841.
- Teitelbaum, J., Morello, G., & Goudie, S. (2020). Nutritional Intervention in Chronic Fatigue Syndrome and Fibromyalgia (CFS/FMS) A Unique Porcine Serum Polypeptide Nutritional Supplement. The Open Pain Journal, 13(1).
- Nie, T., Wang, W., Liu, X., Wang, Y., Li, K., Song, X., … & He, Z. (2021). Sustained Release Systems for Delivery of Therapeutic Peptide/Protein. Biomacromolecules, 22(6), 2299-2324.
- https://www.sciencedaily.com/releases/2020/05/200511112545.htm
- Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Drug Dispos. 1996; 33:285–301.
- Wallace, B. M., & Lasker, J. S. (1993). Stand and deliver: getting peptide drugs into the body. Science, 260(5110), 912-913.
- https://news.drgator.ufl.edu/
- Moroz, E., Matoori, S., & Leroux, J. C. (2016). Oral delivery of macromolecular drugs: Where we are after almost 100 years of attempts. Advanced drug delivery reviews, 101, 108-121.
- Fonte, P., Araújo, F., Reis, S., & Sarmento, B. (2013). Oral insulin delivery: how far are we?. Journal of diabetes science and technology, 7(2), 520-531.
- US Food and Drug Administration. (2017). Inactive ingredient search for approved drug products. FDA Database.
- Griesser, J., Hetényi, G., Moser, M., Demarne, F., Jannin, V., & Bernkop-Schnürch, A. (2017). Hydrophobic ion pairing: Key to highly payloaded self-emulsifying peptide drug delivery systems. International journal of pharmaceutics, 520(1-2), 267-274.
- Meaney, C. M., & O’driscoll, C. M. (2000). A comparison of the permeation enhancement potential of simple bile salt and mixed bile salt: fatty acid micellar systems using the CaCo-2 cell culture model. International journal of pharmaceutics, 207(1-2), 21-30.
- https://doctorpaulvin.com/blog/heal-and-maximize-your-performance-with-bpc-157/
- Dalmasso, G., et al. (2008). PepT1-Mediated Tripeptide KPV Uptake Reduces Intestinal Inflammation. Gastroenterology, 134(1), 166–178. DOI: 10.1053/j.gastro.2007.10.026
- Mehta D, Granstein R, D: Immunoregulatory Effects of Neuropeptides on Endothelial Cells: Relevance to Dermatological Disorders. Dermatology 2019;235:175-186. doi: 10.1159/000496538
- Van de meent H, Hamers FP, Lankhorst AJ, Joosten EA, Gispen WH. Beneficial effects of the melanocortin alpha-melanocyte-stimulating hormone on clinical and neurophysiological recovery after experimental spinal cord injury. Neurosurgery. 997;40(1):122-30
- Sinha PS, Schiöth HB, Tatro JB. Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered alpha-MSH. Brain Res. 2004;1001(1-2):150-8
- https://thegutinstitute.com/products/integrative-peptides-can-t-weight
- https://www.drambernd.com/peptide-store/cerebropep
- https://www.drambernd.com/peptide-store/tb-500
- Haddadzadegan, S., Dorkoosh, F., & Bernkop-Schnurch, A. (2022). Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Advanced Drug Delivery Reviews, 114097.
- Haddadzadegan, S., Dorkoosh, F., & Bernkop-Schnurch, A. (2022). Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Advanced Drug Delivery Reviews, 114097.
- Arbit, E., & Kidron, M. (2017). Oral insulin delivery in a physiologic context. Journal of diabetes science and technology, 11(4), 825-832.
- Hämmerle, S. P., Mindeholm, L., Launonen, A., Kiese, B., Loeffler, R., Harfst, E., … & John, M. R. (2012). The single dose pharmacokinetic profile of a novel oral human parathyroid hormone formulation in healthy postmenopausal women. Bone, 50(4), 965-973.
- Tankó, L. B., Bagger, Y. Z., Alexandersen, P., Devogelaer, J. P., Reginster, J. Y., Chick, R., … & Christiansen, C. (2004). Safety and efficacy of a novel salmon calcitonin (sCT) technology‐based oral formulation in healthy postmenopausal women: acute and 3‐month effects on biomarkers of bone turnover. Journal of bone and mineral research, 19(9), 1531-1538.
- Gleeson, J. P., Fein, K. C., & Whitehead, K. A. (2021). Oral delivery of peptide therapeutics in infants: Challenges and opportunities. Advanced drug delivery reviews, 173, 112-124.
- Brown, T. D., Whitehead, K. A., & Mitragotri, S. (2020). Materials for oral delivery of proteins and peptides. Nature Reviews Materials, 5(2), 127-148.
- Anselmo, A. C., Gokarn, Y., & Mitragotri, S. (2019). Non-invasive delivery strategies for biologics. Nature Reviews Drug Discovery, 18(1), 19-40.
- Lewis, A. L., McEntee, N., Holland, J., & Patel, A. (2022). Development and approval of rybelsus (oral semaglutide): ushering in a new era in peptide delivery. Drug delivery and translational research, 12(1), 1-6.
- Beauchesne, P. R., Chung, N. S., & Wasan, K. M. (2007). Cyclosporine A: a review of current oral and intravenous delivery systems. Drug development and industrial pharmacy, 33(3), 211-220.
- Heinemann, L., & Jacques, Y. (2009). Oral insulin and buccal insulin: a critical reappraisal. Journal of diabetes science and technology, 3(3), 568-584.
- Maher, S., & Brayden, D. J. (2012). Overcoming poor permeability: translating permeation enhancers for oral peptide delivery. Drug Discovery Today: Technologies, 9(2), e113-e119.
- Morales, J. O., & Brayden, D. J. (2017). Buccal delivery of small molecules and biologics: of mucoadhesive polymers, films, and nanoparticles. Current opinion in pharmacology, 36, 22-28.
- Samson, S. L., Nachtigall, L. B., Fleseriu, M., Molitch, M. E., Giustina, A., Ludlam, W. H., … & Strasburger, C. J. (2021). One-year outcomes of the open-label extension of CHIASMA OPTIMAL, a phase 3 study of oral octreotide capsules in acromegaly. Journal of the Endocrine Society, 5(Suppl 1), A515.
- Dossche, L., Michelet, R., De Bruyne, P., Van Herzeele, C., Gasthuys, E., Rittig, S., … & Walle, J. V. (2021). Desmopressin oral lyophilisate in young children: new insights in pharmacokinetics and pharmacodynamics. Archives of Disease in Childhood, 106(6), 597-602.
- Juárez, B. A., Fleischmann-de la Parra, P., Rayo-Mercado, K. T. E., & Ponce-Lopez, T. (2021). Comparison between insulin delivery methods: subcutaneous, inhaled, oral, and buccal. Proceedings of Scientific Research Universidad Anáhuac. Multidisciplinary Journal of Healthcare, 1(1), 62-71.
- Kintzing, J. R., & Cochran, J. R. (2016). Engineered knottin peptides as diagnostics, therapeutics, and drug delivery vehicles. Current opinion in chemical biology, 34, 143-150.
- Bahman, F., Greish, K., & Taurin, S. (2019). Nanotechnology in insulin delivery for management of diabetes. Pharmaceutical nanotechnology, 7(2), 113-128.
- Vass, P., Démuth, B., Hirsch, E., Nagy, B., Andersen, S. K., Vigh, T., … & Marosi, G. (2019). Drying technology strategies for colon-targeted oral delivery of biopharmaceuticals. Journal of Controlled Release, 296, 162-178.
